In History
Wales Becomes First in U.K. to Approve Sativex on the NHS
Following the approval from the Health Minister and recommendation from the All Wales Medical Strategy Group (AWMSG), Sativex will now be available through the publicly-funded healthcare system in Wales. This makes the Welsh country the first in the United Kingdom to fund the effective treatment for patients and make it available by prescription.
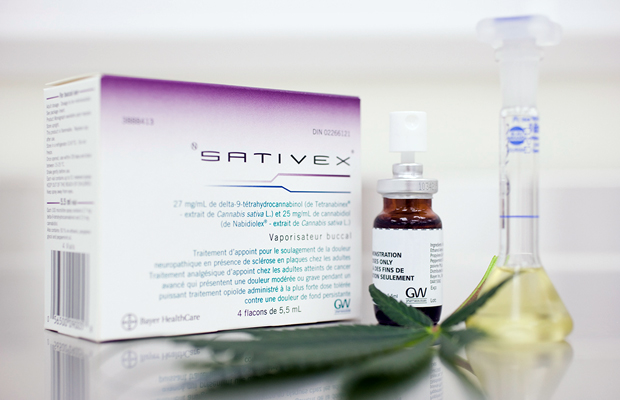
Sativex is a cannabis-based oral mouth spray developed by a company in the United Kingdom called GW Pharmaceuticals. It’s commonly used to treat symptoms like muscles spasms, overactive bladder pain and other ailments commonly associated with multiple sclerosis. Unlike synthetic cannabis products, Sativex is a mixture of cannabis compounds from the plant and effectively acts as a tincture.
Although the treatment has been licensed for use since 2010, this is the first year it has undergone clinical assessment and will be made routinely available to patients. Before now, physicians haven’t been able to prescribe the spray and patients only had access to the treatment on an ad hoc basis. Currently, just one in 50 patients is funded the drugs via the Wales National Health Service (NHS).
“For some time we’ve been aware of people in Wales paying privately for this licensed treatment,” said Sally Hughes, Program Director for Policy at the Multiple Sclerosis Society. “This decision should make life a lot easier for them.”
Mark Drakeford, the health minister for health and social services at the NHS was inspired by AWMSG’s glowing appraisal that insisted that Sativex be made available as treatment for multiple sclerosis. The group acts as an advisory board that provides guidance on medicine management and prescription to the government. Following their approval, he decided to make the treatment publicly available.
Interestingly, this decision comes after the National Institute for Health and Care Excellence (NICE) rejected Sativex as treatment for multiple sclerosis in April in a draft of their recent guidelines.
“Sativex has been licensed as safe and effective for people with MS, and for many people it’s their only viable treatment option left. Despite this, NICE has rejected this medicine for use. It means people are either left with the daily battle of painful symptoms, or face financial strain as a result of funding the treatment themselves,” Hughes said.
If their judgement remains in October, when the final guidelines are expected, Sativex won’t be available in England, Scotland and Northern Ireland — only Wales.
What do you think about the changes in Wales? Tell us in the comments below.